Cleanroom monitoring: How measurement technology helps to ensure air quality
In cleanrooms where sensitive processes take place, a controlled atmosphere is crucial. Cleanroom monitoring is used to monitor the air quality in such environments and ensure that particles and contaminants remain below a certain limit. Measurement technology plays an essential role here, as it can provide precise data on factors such as air pressure, temperature, humidity and particle size. This enables early detection of deviations and allows operators to take appropriate measures to maintain air quality. By using advanced measurement technology, cleanroom monitoring can make an important contribution to ensuring process safety and product quality in sensitive environments).
During production and storage, but also in research and development in the pharmaceutical industry, particular attention is paid to complete and tamper-proof data recording and documentation. According to FDA Part 11 regulations Data acquisition and monitoring systems in cleanrooms must be validated in accordance with GAMP/GMP guidelines. Measured values and limit values for parameters such as temperature, humidity, particle count and pressure must be recorded, monitored and archived reliably and tamper-proof. Via a Audit trail all user interventions relevant to the process are also saved.
Application highlights of cleanroom monitoring
- Tamper-proof recording of all measured values in cleanrooms and pharmaceutical environments
- Autonomous data storage in the Delphin recording device parallel to the PC
- Flexible set-up of monitoring functions with e-mail, fax, SMS or switching outputs
- Individually customizable process visualization and reports with ProfiSignal
- Scalable system for small and large solutions
Practical example of cleanroom monitoring
Long-term stability of active pharmaceutical ingredients
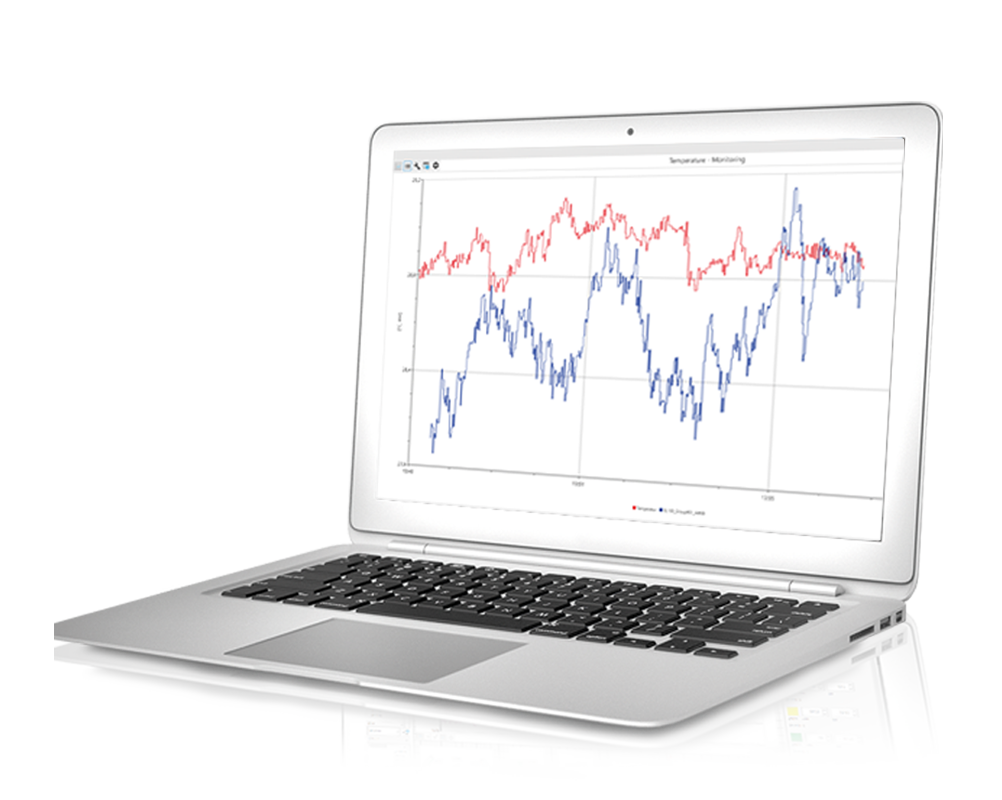
Testing the long-term stability of active pharmaceutical ingredients places special demands on data collection. The active ingredients are stored in climate chambers and freezers, where environmental conditions must be recorded, archived and documented. Changes to operating parameters such as target temperatures or limit values as well as the ambient conditions must be archived reliably and tamper-proof. User and alarm management are integrated for this purpose. All alarms are automatically logged on a weekly basis and parameter changes are logged monthly with comments. All digital documents are stored redundantly. Many hospital pharmacies have been equipped with Delphin clean room monitoring systems.
Typical areas of application
of cleanroom monitoring
- Part 11 compliant pharmacy monitoring
- Clean room monitoring
- Distributed systems for pharma monitoring tasks
- Monitoring of production facilities according to FDA
- Integrated total monitoring solutions in pharmaceutical production
- Integrated alarm systems
Hardware and software for your measurement and testing solution






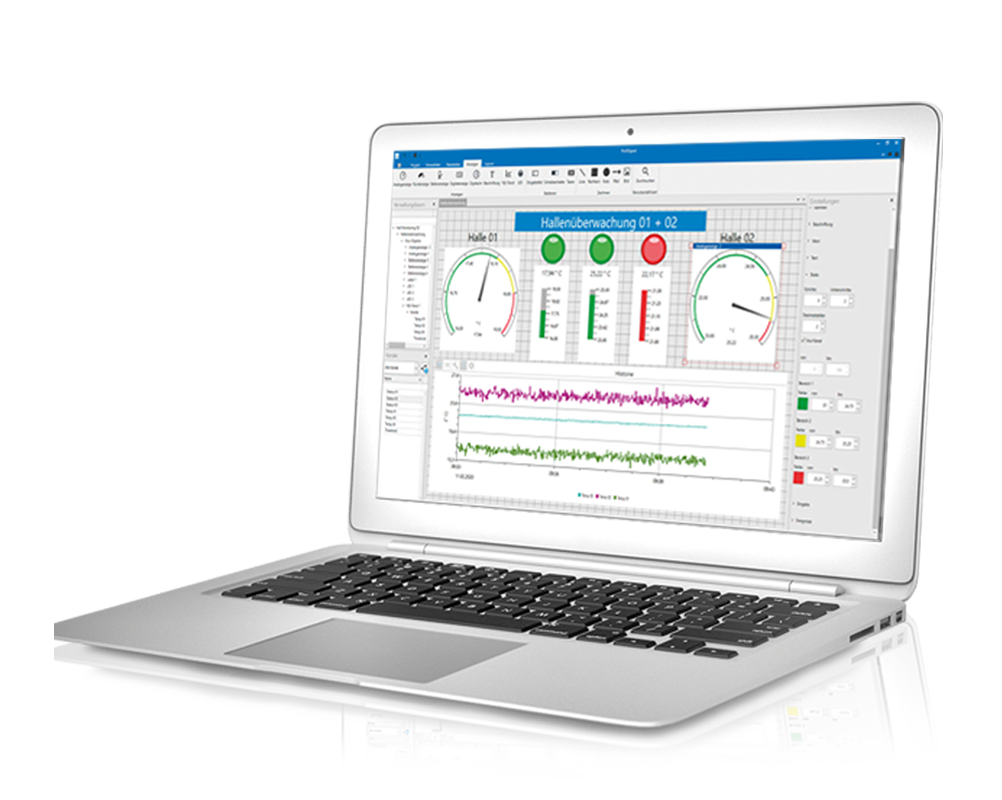





Flexible, scalable and expandable solution for seamless data acquisition and monitoring
The Loggito device is a compact data logger system designed for the acquisition and recording of measurement data. It has a variety of analog and digital inputs to connect different sensors and signals. The device records the data with high accuracy and stores it on an internal memory or external storage media such as SD cards.
To the product
Data acquisition and analysis
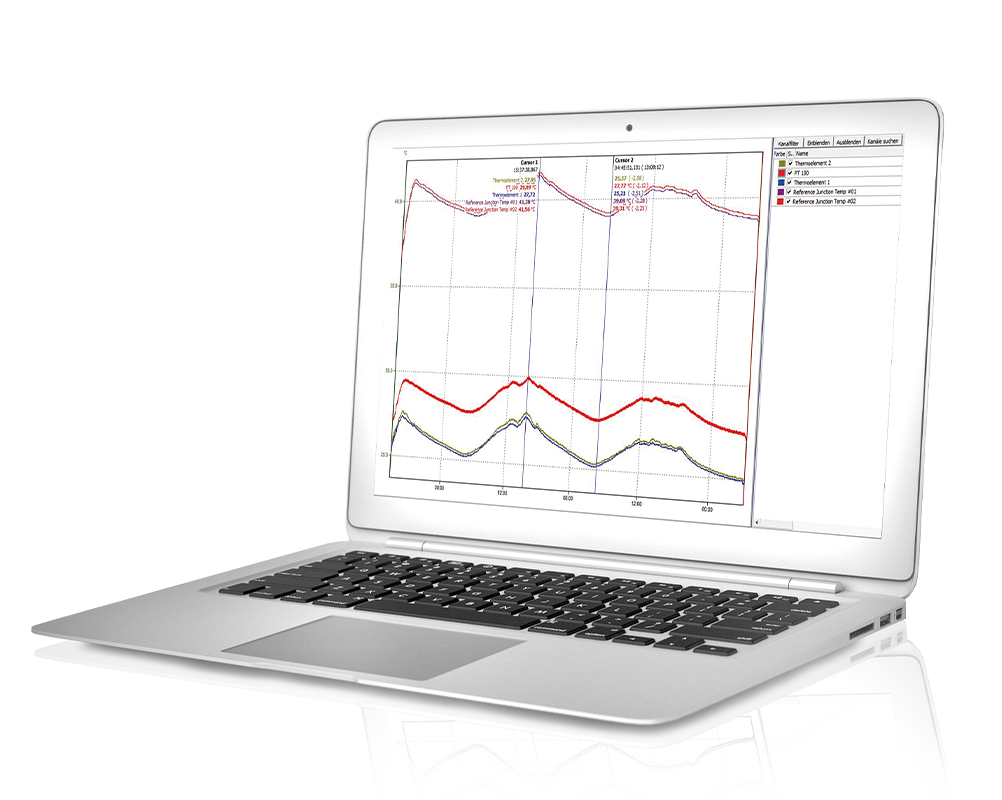
With ProfiSignal Go you can display online data from the Message and Expert hardware in diagram form. Numerous analysis functions, such as zoom, cursor, movable axes, flags, ASCII export and calculation channels, enable fast display of measured values and post-processing.
To the product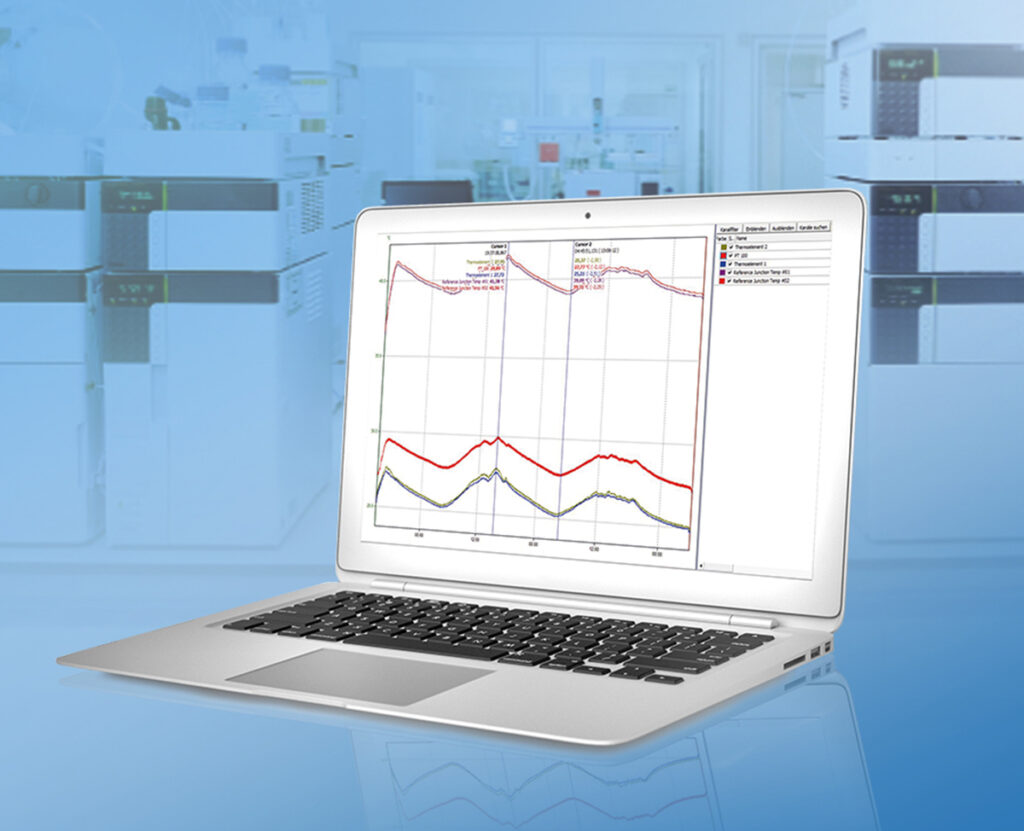
Precisely record, autonomously store, automatically transmit and evaluate measured values.
The FPGA-based Expert Logger device is particularly powerful and enables the processing of up to 46 analogue input channels, both at low and high sampling rates. Measured values can be precisely recorded, autonomously stored and automatically transferred to the internet or PC via USB, LAN or WLAN and evaluated.
To the product
Operating and monitoring
ProfiSignal Basic combines the functions of ProfiSignal Go with operating and monitoring elements of process visualisations, e.g. digital and analogue displays, switches, buttons and signal lamps. With Basic, you can create everything from simple visualisation diagrams to complex visualisation systems.
To the product
Modular measuring, monitoring and automation in one device.
The ProfiMessage D device is a state-of-the-art data logger system that has been specially developed for industrial data acquisition and analysis. It offers a wide range of functions such as the recording and storage of measurement data, monitoring of process parameters and alarms in the event of deviations.
To the product
Automate and control
ProfiSignal Klicks supplements ProfiSignal Basic with functions for process automation. Structural diagrams for mapping the process as well as a script language (completely operable with the mouse) enable even the non-informatician to create complex test stand and automation applications with automatic report generation.
To the product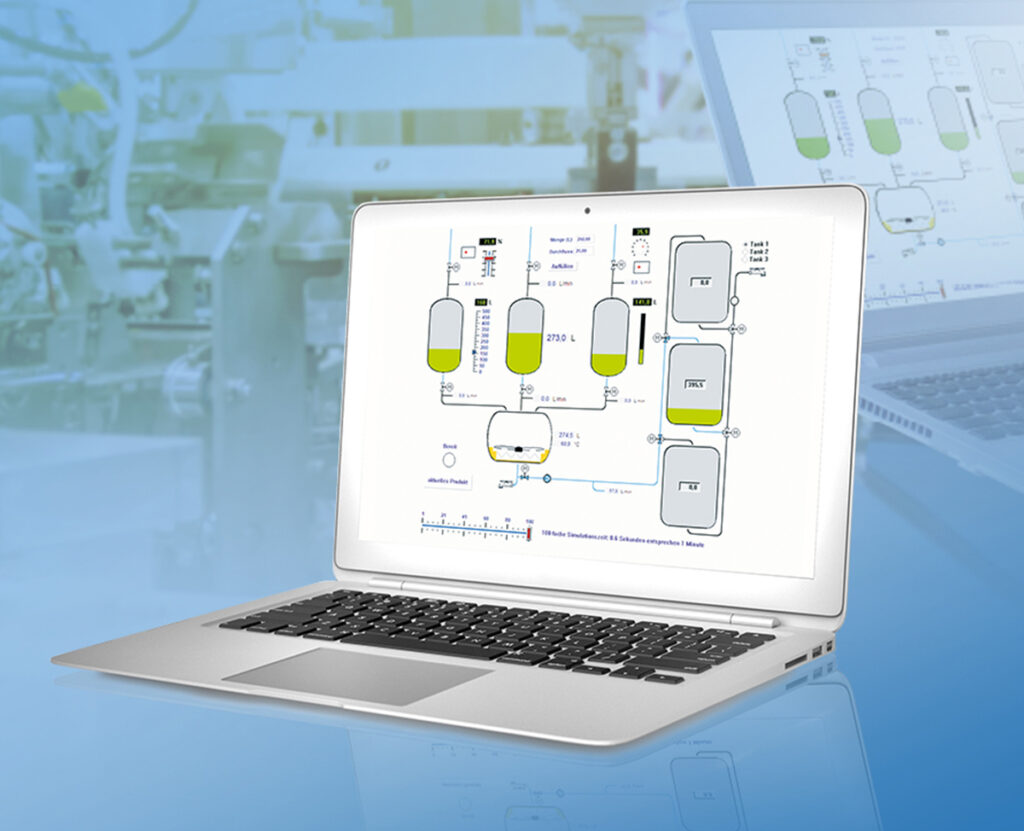
State-of-the-art processor technology and extreme precision for vibration measurement.
The Expert Vibro device is a powerful vibration analysis device that has been specially developed for monitoring and analyzing machine and system conditions. It enables vibration data to be recorded and evaluated in order to detect potential problems or signs of wear at an early stage.
To the product
Fast project planning and analysis of measurement data
With ProfiSignal 20 Go you can visualise your measurement data in just a few steps, both online and offline, in various diagram types, monitor, analyse, archive as a measurement file or export directly in the appropriate file format.
To the product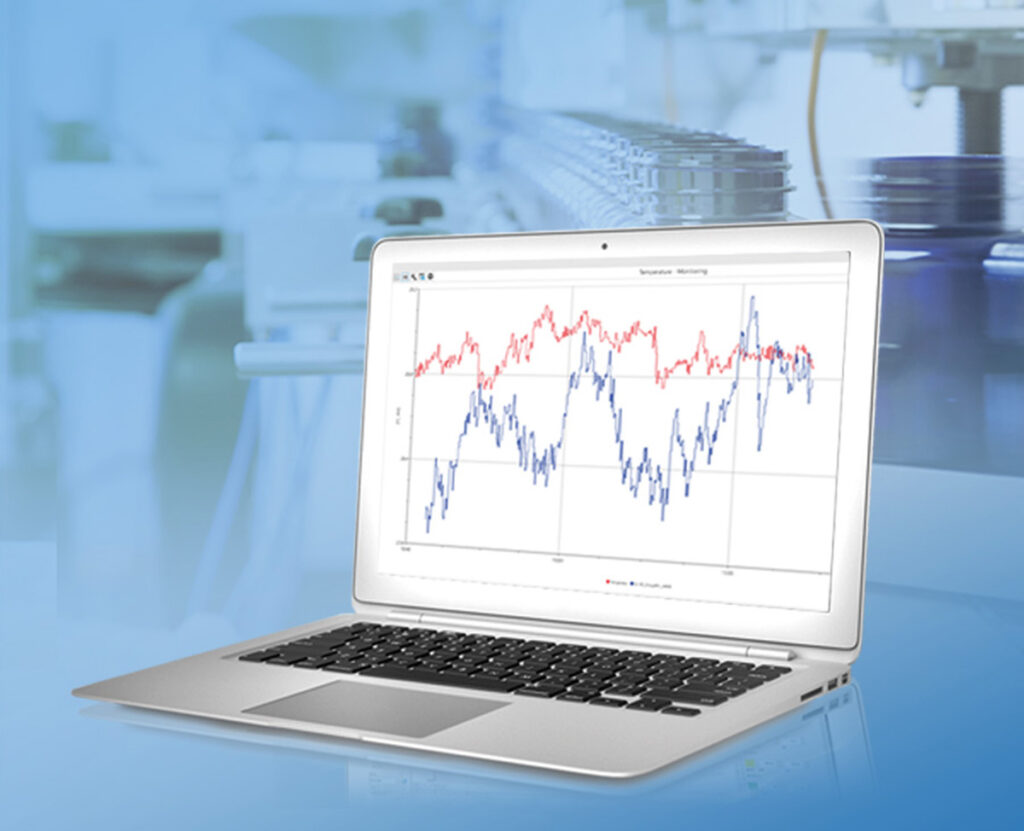
Intelligent data recorder for synchronous recording of transient and periodic events.
The Expert Transient device enables the recording and evaluation of transient signals over a certain period of time and provides important information for the optimization of processes and systems. The device is characterized by its high accuracy, fast sampling rate and simple operation.
To the product
Visualisation and operation
With ProfiSignal 20 Basic you can create individual diagrams using a wide range of operating and monitoring elements. Both continuous processes (e.g. production data acquisition) and discontinuous measurement tasks (e.g. test measurements) can be visualised, operated and monitored without any programming effort. You can create the operating and monitoring diagrams by assembling and configuring the prefabricated elements.
To the product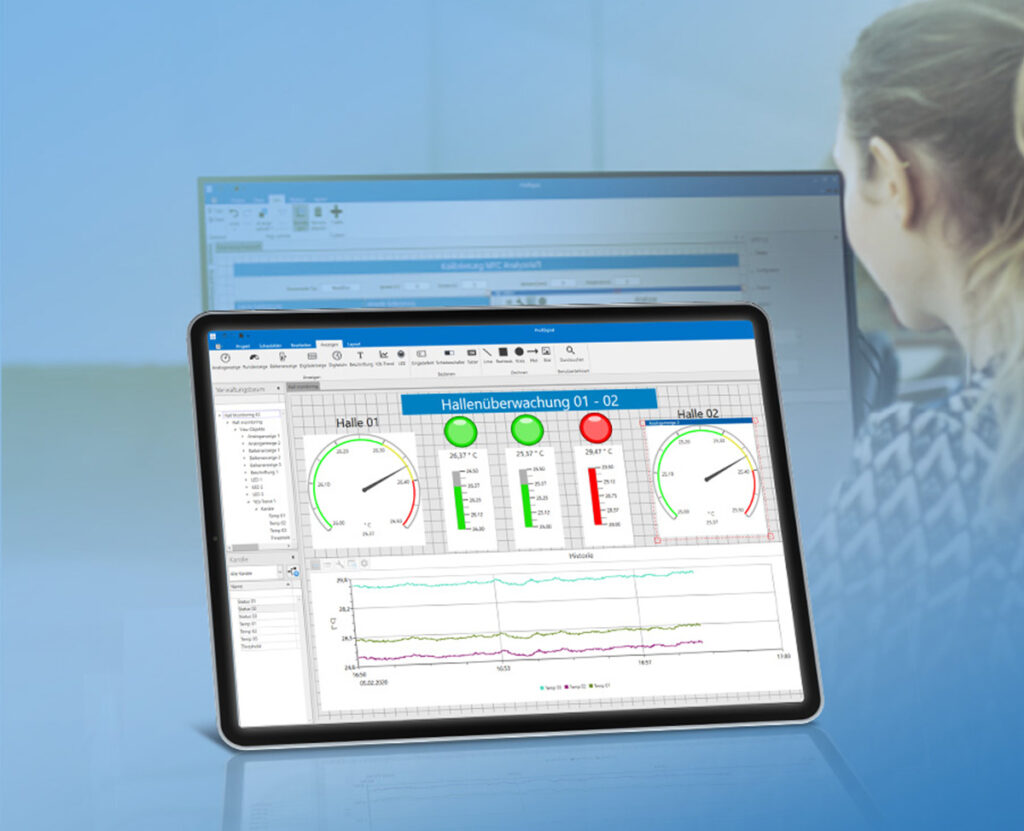
Modular system for use as a measuring, control and monitoring device.
The ProfiMessage devices are a modular, expandable and easy-to-use system for measurement data acquisition, monitoring and automation of machines, systems or test benches. ProfiMessage can be used wherever measured values need to be recorded quickly, precisely and electrically isolated, as well as intelligently pre-processed or monitored.
To the product
Central measurement data management
The Delphin Data Center is the solution used for the worldwide measurement networking and control of plants, machines and test stands.
To the product
PC-supported data acquisition and test stand
The Expert Key device is a measuring and control device that can be used in various applications. It enables the acquisition and evaluation of measurement data as well as the control of processes. The device has various interfaces and can be connected to a wide range of sensors and actuators. It is often used in automation technology, mechanical engineering and research.
To the product
Compact and flexible measurement data laboratory with precision measurement technology.
The LoggitoLab device is ideal for use in laboratory environments, research projects, quality control and other applications where precise data acquisition and analysis is required. It offers a reliable and user-friendly solution for monitoring and recording measurement data.
To the product
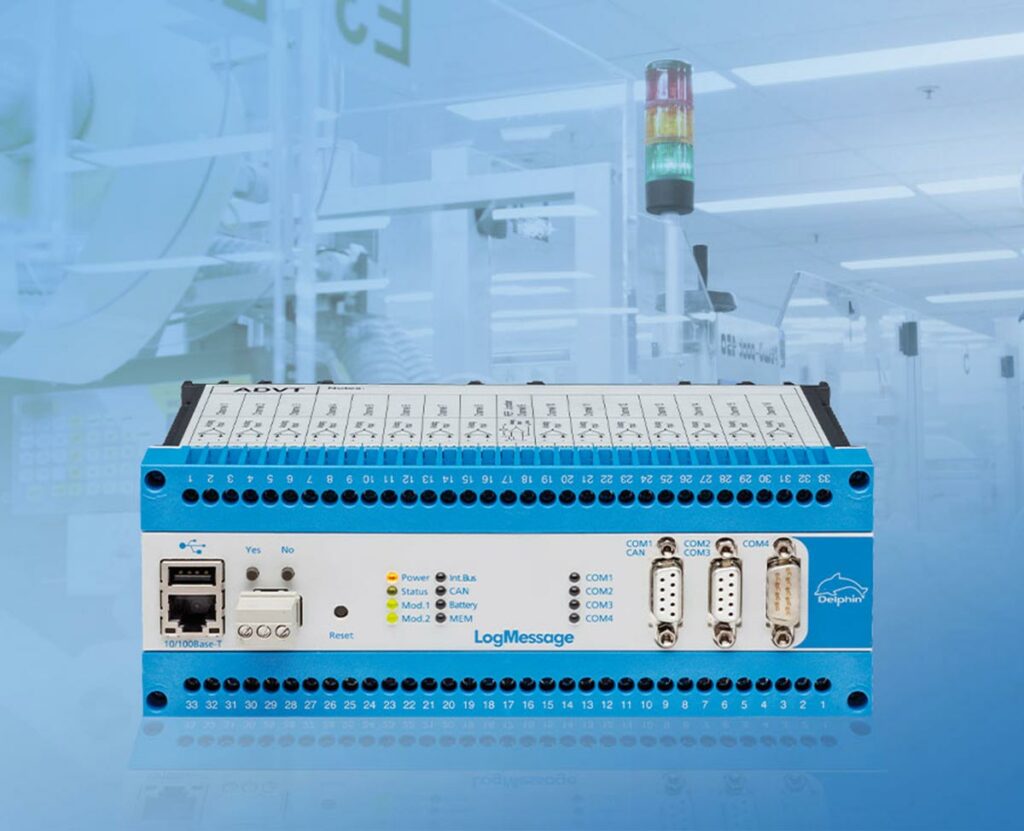
Test stand for measurements with potential using an internal data memory.
The LogMessage device is a powerful data logger system designed for the reliable acquisition and recording of measurement data. It offers a variety of analog and digital inputs to connect different sensors and signals. The device records the data with high accuracy and saves it to an internal memory or external storage media.
To the product